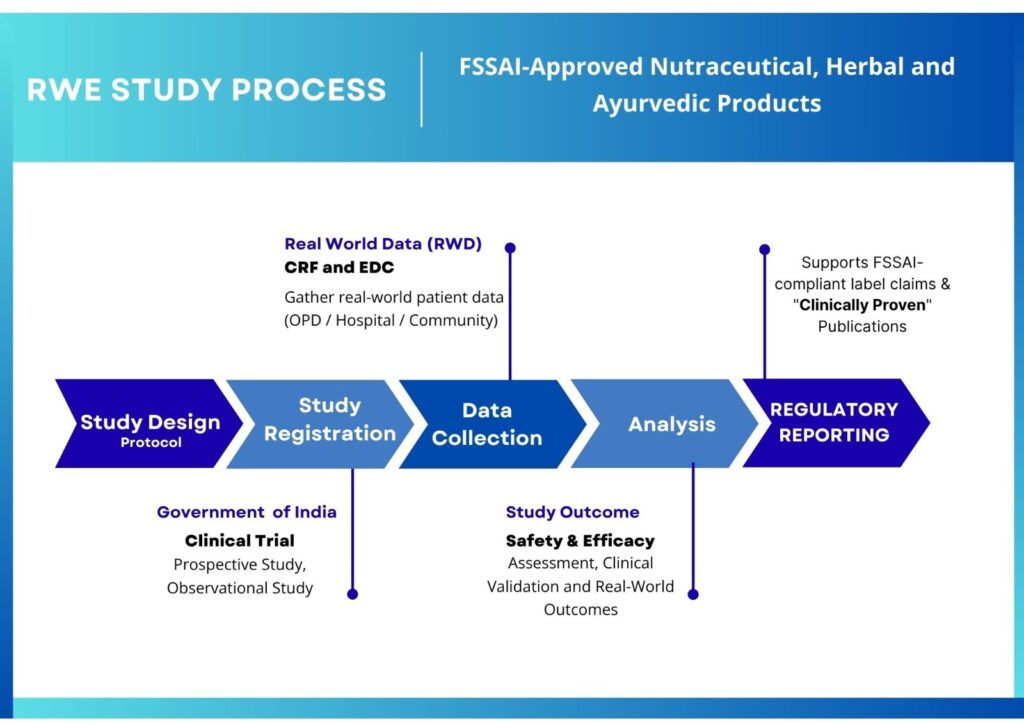
Real-World Evidence (RWE) Studies
Transforming Real-World Data into Actionable Evidence
At InventaLife, we recognize the growing importance of Real-World Evidence (RWE) in shaping clinical, regulatory, and market access decisions—especially in the domains of Ayurveda and Nutraceuticals, where traditional use meets modern science.
Our RWE services are designed to generate credible, real-life data on product safety, effectiveness, adherence, and outcomes outside the controlled environment of randomized clinical trials. By utilizing data collected from real-world sources such as electronic health records (EHRs), patient registries, observational studies, and post-marketing surveillance, we help sponsors validate the true value of their products in actual healthcare settings.
Ayurvedic Trials
Evidence-Based Clinical Research for Traditional Formulations
At InventaLife, we specialize in conducting Ayurvedic clinical trials that merge ancient healing wisdom with modern scientific rigor. With the increasing demand for validated herbal therapies and alternative medicines, we provide comprehensive research solutions to scientifically evaluate the safety, efficacy, and tolerability of Ayurvedic formulations.
Our approach is rooted in evidence-based medicine while respecting the traditional principles of Ayurveda. We design trials that meet regulatory expectations set by authorities like the Ministry of AYUSH, CDSCO, and global bodies, ensuring your products are ready for both national and international markets.
Our Ayurvedic research capabilities include
- Designing and conducting observational, interventional, and comparative studies
- Developing protocols that incorporate Ayurvedic diagnostic principles and endpoints
- Standardization and authentication of herbal raw materials and formulations
- Clinical data collection, monitoring, and analysis tailored for Ayurveda-based interventions
- Compliance with ICH-GCP and Good Clinical Practice for Ayurveda Guidelines
- Preparation of AYUSH regulatory dossiers and documentation for marketing approvals5Publication support for journals focused on integrative and complementary medicine
We work closely with Ayurvedic practitioners, research institutes, and regulatory experts to ensure holistic and credible study execution. Whether your product is a classical formulation or a proprietary blend, InventaLife helps you build scientific credibility and market acceptance through ethical and robust clinical research.
Let us help you bring traditional remedies to global platforms with the strength of validated evidence.
We specialize in
Nutraceutical Trials
Scientific Validation for Dietary Supplements and Functional Foods
At InventaLife, we offer specialized nutraceutical clinical trial services designed to assess the safety, efficacy, tolerability, and real-world effectiveness of dietary supplements, functional foods, and health products. With growing consumer demand and increased regulatory scrutiny, it is essential to support health claims with strong clinical evidence.
Our end-to-end support helps nutraceutical companies meet compliance standards outlined by FSSAI, ICMR, and international regulatory authorities, while also ensuring that trial outcomes are suitable for publication and product marketing.
Our expertise in nutraceutical research includes:
- Protocol development specific to nutritional and wellness endpoints
- Design and execution of pilot, bioavailability, and multi-center efficacy trials
- Target population recruitment (e.g., general wellness, lifestyle disorders, age-specific groups)
- Design and execution of pilot, bioavailability, and multi-center efficacy trials
- Target population recruitment (e.g., general wellness, lifestyle disorders, age-specific groups)
- Standardized data collection methods to track measurable health improvements
- Assessment of biomarkers, tolerability, and quality of life metrics
- Real-world evidence (RWE) generation using observational and post-marketing studies
- Regulatory documentation for FSSAI approval and global market expansion
- Preparation of scientific publications and white papers to support marketing claims
We understand the unique challenges involved in studying non-pharmaceutical products and offer a flexible, scientifically rigorous framework to validate them. Our integrated team of clinical researchers, nutrition experts, and
regulatory professionals ensures that your product is backed by credible, consumer-trusted data.
Partner with InventaLife to bring evidence-based confidence to your nutraceutical innovations.
Our team works closely with researchers, clinicians, and regulatory authorities to design RWE studies that are statistically sound, ethically conducted, and publication-ready, helping you bridge the gap between traditional products and modern medical evidence.
Let InventaLife guide your product from traditional use to scientific validation.
